Understanding Human-Centered Design
“If you think good design is expensive, you should look at the cost of bad design,” said Dr. Ralf Speth, CEO of Jaguar Land Rover. While the medical device industry is not the same as the automobile industry, the sentiment still applies. Not only can a bad design result in lost profit, wasted product, and extra effort, but a poorly designed medical device can put lives at risk. Understanding user needs and taking a human-centric approach to design is critical for the success and safety of any medical or drug delivery device.
What is Human-Centered Design?
Human-centered design (HCD) is an inclusive and collaborative approach to design that seeks to place the end user at the center of the design process. This is also sometimes referred to as user-centered design (UCD). The International Organization for Standardization defines this further in the ISO 9241-210 standard, explaining human-centered design as “an approach to interactive systems development that aims to make systems usable and useful by focusing on the users, their needs and requirements, and by applying human factors/ergonomics, and usability knowledge and techniques. This approach enhances effectiveness and efficiency, improves human well-being, user satisfaction, accessibility and sustainability; and counteracts possible adverse effects of use on human health, safety and performance.”
Human Factors in Medical Device Design & Development
Human factors refers to the relationship between the human beings and the systems with which they interact. Human factors engineering (HFE) is the discipline that applies these human factors to the design process. Understanding human factors is vital when making medical devices. Not only does human-centric design lead to a better user experience, but it helps to reduce risk, minimize user error, and protect patients from adverse health events.
In the US, the FDA has become increasingly insistent that medical device manufacturers incorporate human factors engineering into the medical device design and development process. They issued an official guidance document for applying human factors and usability engineering to medical devices in 2016. The FDA also drafted similar guidance regarding human factors considerations for combination products. The guidance outlined in these documents is suggested, but not required, by the FDA to assist medical device manufacturers in improving the design of devices to minimize potential user error and reduce harm.
Not all medical device companies follow a human-centered design process. They may design a device to meet technical specifications and then consider usability design as an afterthought. Then these same development teams are frustrated when they observe users operating a device “wrong” or in a way that wasn’t intended, without considering the possibility that maybe they designed it “wrong” by not paying attention to human factors early on. To incorporate human factors into the medical device design and development process, the device’s end-user interface must be scrutinized throughout the entirety of the design cycle, starting in the predesign phase. Implementing a detailed human factors plan and following the steps for human-centered design can help ensure that human factors and user needs are considered.
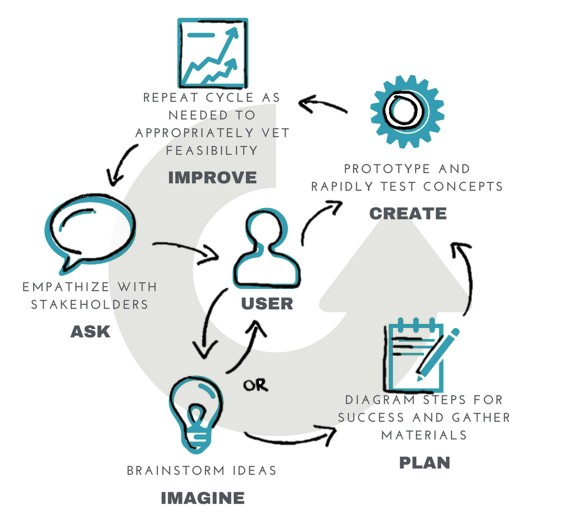
Gilero’s User-Centered Design Cycle
There are typically three standard phases for human-centered design: inspiration (understanding the end user), ideation (brainstorming and identifying solutions), and implementation (bringing solution to life and launching in the market). At Gilero, our human factors engineers have created a more detailed “design thinking cycle” for user-centered design, which we follow as an adaptation of these three phases. We ensure that every aspect of our medical device design process revolves around the end user.
The Cost of Ignoring Human Factors
Employing human-centered design is critical in medical device design and development. But what happens when those responsible for product development fail to gather user feedback? Consequences of a bad design range from minor inconveniences, like pulling on a door that must be pushed open, to the worst-case scenario of causing serious injury or death. It’s estimated that medical errors cause approximately 250,000 deaths in the US each year. Many of these deaths, as well as non-fatal injuries, can be traced back to a misuse of medical equipment. These unintentional user errors can occur because user needs and current workflow do not match up with the device’s user interface. Medical devices that are designed without usability in mind can be deemed unsafe, prone to user error, difficult to use, or difficult to learn to use. According to the FDA, applying human factors to medical devices, and making design modifications as necessary, is an effective way to reduce these types of user errors. If investing in human-centered design seems expensive, consider the potential costs of a poorly designed device.
Human-Centered Design at Gilero
At Gilero, we follow a human-centric approach to medical device design. The first step we recommend for any medical or drug delivery device project is to create a detailed human factors plan. Gilero will follow that plan in parallel to product development, ensuring that the product design meets end-user needs. We have a team of dedicated human factors engineers that routinely conduct market evaluations, user research, formative studies, and summative studies. Our onsite usability lab allows Gilero engineers to observe simulated use studies and gain user feedback in real time. This helps ensure that device designs are intuitive and can be easily incorporated into current UX workflows. By bringing user needs into focus early within the design and development process, we deliver devices that are readily embraced by users and can be operated safely in real-world conditions. To learn more about Gilero’s design and development services, including human factors, contact us today!
Ready to turn your idea for a medical or ocular drug delivery device into a reality?
Talk with an expert today.


