Proof of Concept for Medical Devices
What is Proof of Concept (POC) and How is it Applied to Medical Device Development?
When it comes to medical device development and manufacturing, Proof of Concept (POC) refers to the initial stage in the medical device development process where feasibility and potential viability of the device concept are assessed.
Essentially, proof of concept (POC) serves as the crucial bridge between visionary medical device concepts and practical reality. This process involves breaking down ideas into fundamental components and crafting prototypes that undergo rigorous testing and refinement. Collaborative evaluation by engineers, designers, and medical experts ensures functionality and usability with intended clinical outcomes.
Through simulated real-world scenarios, any necessary adjustments are made, transforming the prototype into a viable embodiment of the original concept. This meticulous phase informs subsequent development stages, guiding design enhancements, production strategies, and regulatory considerations, ultimately leading to innovative solutions that reshape patient care.
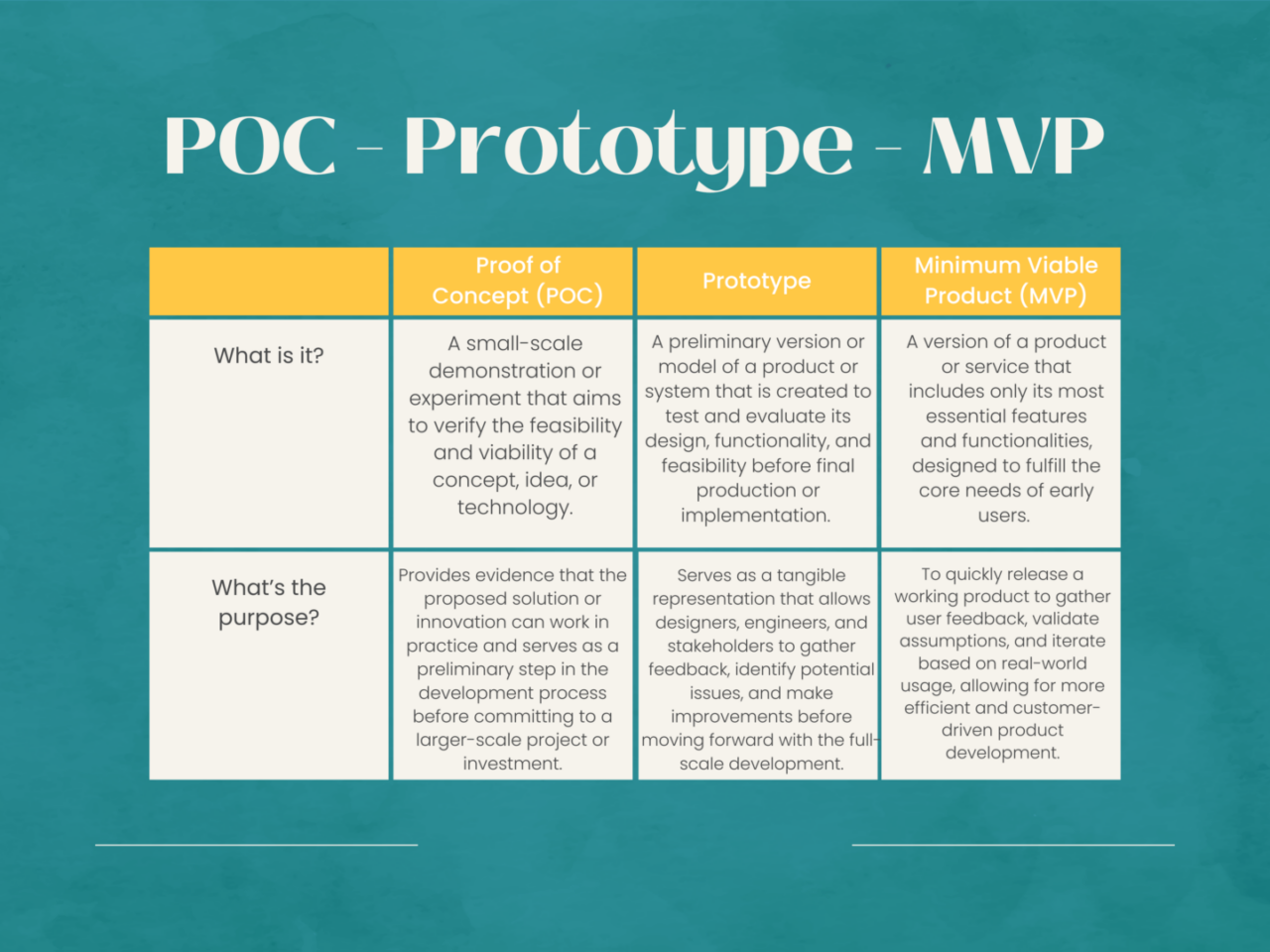
Proof of Concept vs. Prototypes & MVP
Prototypes
The difference between prototypes and POC is outlined within their purpose and stage during the medical device development process. A POC is conducted in the early stages of development to assess the feasibility of the concept or technology of the device. It involves demonstrating the basic functionality of a concept via small scale tests and simulations.
Prototypes are created in a more advanced stage within the medical device development process. It is a tangible and working model of the final device design. These prototypes are created to test and refine the device to prove its functionality, user experience, and design.
Both POC and prototypes are important steps in the medical device development process but serve different purposes. The POC helps determine the feasibility of an idea while a prototype focuses on refining and testing the design of the device. Both of these stages contribute to ensuring a functional device and mitigate risks along the way.
MVP
In medical device design and manufacturing, the distinction between proof of concept (POC) and minimum viable product (MVP) lies in their respective stages of development and purpose. Proof of concept is the initial exploration phase where the feasibility and viability of an innovative medical device concept are evaluated. It involves creating a prototype to demonstrate the basic functionality and potential of the idea, often without the full range of features.
On the other hand, the minimum viable product represents a more advanced stage, where the core features and functionalities necessary to address a specific medical need are integrated into a refined prototype. While POC validates the concept’s potential, MVP focuses on delivering a tangible solution that can be tested, refined, and validated in real-world scenarios, inching closer to a market-ready medical device.
Proof of Concept Examples in the Medical Industry
- Telemedicine: Before developing a telemedicine platform, a medical technology company might create a POC to demonstrate the basic functionality of virtual consultations, secure data exchange, and user interfaces.
- Wearables Health Devices: A POC could involve creating a wearable device that tracks vital signs and integrates with a mobile application. The prototype would focus on demonstrating the accuracy of the collected data and the device’s usability for patients.
- Implantable (Class III) Medical Devices: a POC could potentially involve creating a prototype of an implantable device to showcase how the device interacts with the body and prove accurate data collection and communication.
Why is Proof of Concept Important?
There are many reasons why a company will elect to curate a POC. They are used to demonstrate the value of the device to key stakeholders, such as investors, board members, and patients. POC also helps engineers pinpoint potential risks and forecast obstacles they may face when fully developing the device. This grants them more flexibility to mitigate these risks before they pose a bigger roadblock in the development process. Additionally, POC provides useful feedback and analysis when it comes to the demand of the market and to validate assumptions of the viability of the device.
Proof of Concept Testing for Medical Devices
At Gilero, we believe that the path from concept to reality begins with a robust proof of concept (PoC) testing phase. This essential step not only validates the viability of your medical device innovation but also sets the groundwork for its successful development. Our PoC testing process is meticulously designed to ensure precision, efficiency, and transformative results.
- Consider the Customer Segment: Understanding your target audience is crucial. Our POC testing dives deep into comprehending the characteristics, preferences, and needs of your intended patient audience. This knowledge helps us to shape the prototype in a way that resonates with end-users and addresses their unique needs.
- Finalizing Testing Goals: The foundation for successful POC testing lies in well-defined goals. Working closely with you, we establish precise and measurable testing objectives. Whether it’s showcasing accuracy, usability, or compatibility, our testing goals are tailored to your vision and the specific challenges your innovation aims to tackle.
- Iterative Feedback and Improvement: POC testing is a collaborative process. As the prototype takes form, we subject it to rigorous evaluation and seek feedback from experts, engineers, and stakeholders. This loop of feedback and refinement ensures continuous enhancement of the prototype’s design, functionality, and performance.
- Proof of Concept Validation: The cumulation of our POC testing involves a comprehensive validation process. Through rigorous testing and analysis, we verify that the prototype meets the predefined goals and aligns with your overarching vision.
How Gilero handles Proof of Concept
At Gilero, POC testing is a critical process in our design and development approach. Building upon early design concepts permits our engineers to identify and execute the necessary early-stage testing to prove feasibility more efficiently. In this phase, we aim to demonstrate that all critical features of the design are viable and will function as intended. Once we determine that the design concept is viable, the design is put under a concept freeze, which means that no more significant changes will be made to the design.
Proof of Concept Capabilities at Gilero:
- Formative human factors studies
- Metrology
- Mechanical performance testing
- Tensile & compression testing
- Gamma irradiation test management
- Environmental conditioning
- Early packaging evaluations
- Vacuum leak testing
- In-circuit testing
- Signal integrity testing
- Power, voltage, resistance & current
- Electronic coupling
Ready to turn your idea for a medical or ocular drug delivery device into a reality?
Talk with an expert today.


