Medical Device Design UX Workflow
UX workflows for medical devices are changing the healthcare industry. Adhering to a workflow that is user-focused is critical to maintaining this level of healthcare innovation. So what should a UX design workflow include, and how can designers keep users at the forefront? Let’s walk through how to ensure your UX workflow will lead to a patient-centered medical device.
What’s the Difference Between UX and UI?
To understand a UX workflow, it’s important to first understand what UX is — and isn’t. UX refers to user experience. More specifically, it’s the experience a user has through interaction with a product or service. UI on the other hand refers to the user interface. This is the asset the user interacts with, be it an app, tool, machine, etc. There is a cause-and-effect relationship between medical user interface design and user experience, since the experience a user will have depends on the quality of the interface. Take mobile medical apps, for instance. An app’s user experience could be determined by how easy it is for the user to navigate, if they can find the information they need, and if they can perform intended functions on the app, like entering patient weight or accessing lab test results. User interface would consist of app characteristics like font size and style, imagery, color choice, page layouts and screen design. Ultimately, one focuses on the person using the product and the other on the product itself.
What Is a UX Workflow?
A UX workflow outlines the steps within your UX process. This could include researching, defining design expectations, wireframing, prototyping, and usability testing. It’s everything you do that pertains to user experience, from ideation to design transfer once you’re ready for production.
The move toward an agile workflow has spread across many industries, and UX is no different. Agile workflows make sense for UX designers. It means that everyone — from designers to developers to researchers and product managers — works cohesively and is on the same page. It also means that product discovery can be a partnership between UX, product management, and software architecture. Plus, UX projects can be broken down and completed in increments, with team members working in tandem and responding to changes based on user testing and feedback that comes out of human factors studies.
UX Workflow for Medical Devices
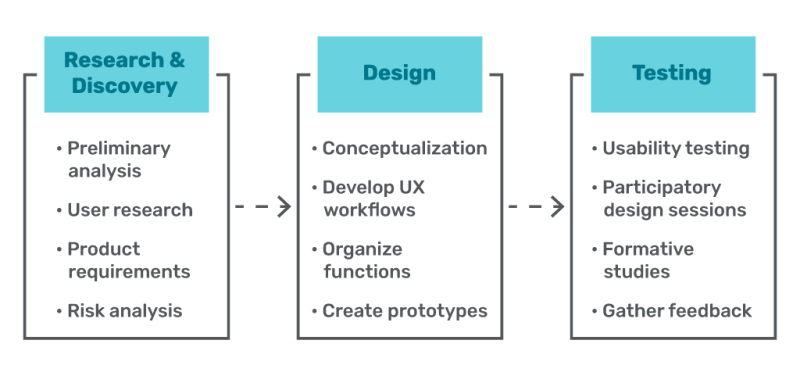
As with most workflows, the UX design workflow for medical devices moves in the general learn – build – measure circle, with safety and proper documentation being priorities throughout the entire process. But let’s break that down. What does a UX workflow actually look like when it comes to designing medical devices?
1. Research & Discovery
To kick off the UX design process, you first want to do a preliminary analysis. Preliminary use specifications can be acquired from available data to structure user research. This draft will be updated throughout the research phase as you gather new information. During this analysis, you’ll likely determine some basics, such as user groups, anticipated user tasks, and typical use environments. With those in mind, you can conduct relevant user research, and use those findings to keep the end user in mind throughout the entire design process. This research often includes things like surveys, focus groups, contextual inquiry, usability testing, and competitor analysis.
2. Design
Now that you’ve gathered data and begun to conceptualize, it’s time to take your product requirements and risk analysis data and organize those requirements into UX workflows. These workflows should be as intuitive as possible and easy for users to interpret so they can properly use the device without making mistakes. One way to do this is to organize by functions and sub-functions, always keeping in mind the end user and ensuring your design provides the most accuracy, convenience, and ease of use for that person. Keep the design of the device, and the accompanying instructions for use (IFU), clear and consistent.
3. Testing
Throughout the design process, you should use prototype devices to conduct any necessary usability testing, participatory design sessions, or formative studies using predetermined criteria. The purpose of these evaluations is to determine the device’s strengths, weaknesses, and unanticipated user errors. After performing these evaluations, make any necessary design adjustments and conduct further evaluations until the results are acceptable. This cycle of making changes through design iterations, rapidly prototyping new designs, and gathering user feedback may need to be repeated several times.
Other Considerations for Medical Device UX Workflow
Understanding the Different Types of Users
It should be obvious that user experience needs to be user-centric at every turn. To design effective medical devices, you must understand the populations who will use them. For example, when conducting user testing for an over-the-counter combination product such as a nasal spray, forming large user groups with characteristics of the general population may be appropriate. However, when evaluating a more sophisticated medical device like a cardiac catheter, user groups should be representative of the physicians and other healthcare providers who will use this type of device.
When it comes to understanding user groups, it’s also important to remember that every decision and change made to a design affects the end user. While you may be inspired to innovate and break the mold to modify a design, this could greatly disrupt how the product fits into current clinician workflows or increase the risk of errors if users don’t fully understand how the device operates.
Understanding the Different Use Environments
The environment in which a product is used is just as important as who is using it. Knowing this information is a crucial part of user experience design. Product design and testing for clinical environments versus home environments will require different approaches.
For example, clinical environments lend the advantage of consistency. Though not all clinical environments perfectly mirror one another, the same fundamentals tend to apply from environment to environment and make designing and testing easier. Optimal use conditions are a priority in clinical environments, so the elements of these environments won’t vary too much. Operating rooms will have their standard conditions, as will intensive care units, emergency departments, MRI suites, doctor’s offices, pharmacies, and so on.
Home environments present unique challenges, however, as conditions can greatly vary. Environmental unpredictability, limitations in caregiver knowledge, and lack of device usability can cause real issues for patients in home-use environments. Because of this, designers must design for the worst-case scenario and try to eliminate as much risk as possible. To do this, you must examine severe, adverse home conditions to ensure even the least capable users (both patients and caregivers) can appropriately use their medical devices. And because patients and caregivers are not only likely to lack medical knowledge, but could also have low levels of education or literacy, it’s imperative that instruction materials for home-use devices be user-friendly.
How UX Workflows Are Changing the Healthcare Industry
The level of user-centered detail that today’s UX design workflow requires is providing opportunities to put patients at the center of their care. User-focused medical devices and advancements in connected smart health solutions open the door to wearables that can track patient wellness from anywhere, home-use drug delivery devices to administer medication, and more readily available care that doesn’t require waiting to be seen by a clinician.
Gilero specializes in end-to-end design, development, and manufacturing for medical and drug delivery devices. Human-centered design is core to our design and development process, and we conduct extensive user research to inform concept development and device design. For more information about our services and capabilities, contact Gilero today.
Ready to turn your idea for a medical or ocular drug delivery device into a reality?
Talk with an expert today.


