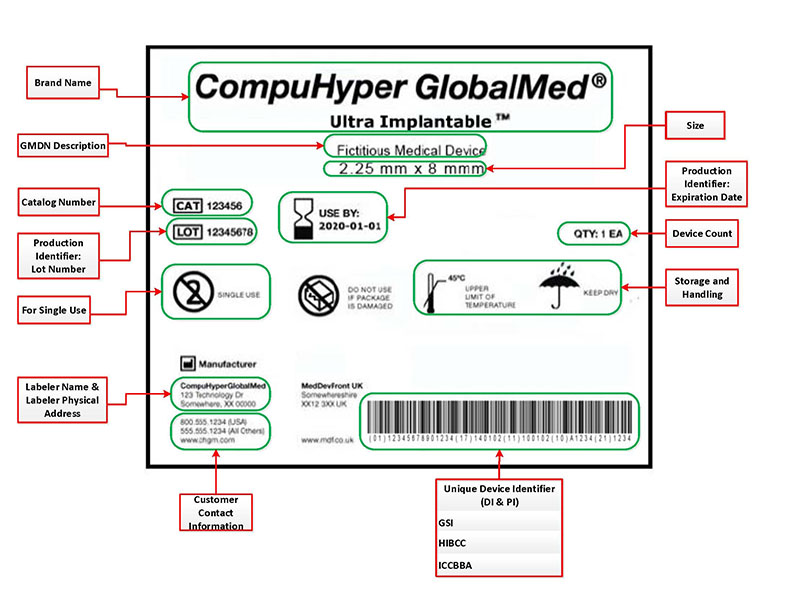
What Is Medical Device Labeling?
Medical device labeling refers to all of the information that is provided to the consumer with the device itself. This includes symbols, warnings, instructions, and control labels applied to the device or incorporated into the design by color coding, printing, machining, or molding.
Medical device labeling also refers to
- Information display on the device’s user interface
- Information printed on the device’s packaging or point-of-sale displays
- Documents provided with the device, including manuals and instructions
Medical device labeling is not just the label on the device, but rather any labels on the pouch, box, instructions for use, etc. The leading international standard for medical device quality systems, ISO 13485, states that a label is any information related to identification, technical description, intended purpose, and proper use of the device.
What Are Medical Device Labeling Requirements?
Medical devices companies that sell their products in the US must adhere to the FDA’s strict medical device labeling requirements. The purpose of these regulations is to help keep medical device users safe. The requirements for labeling a particular medical device depend on what type of device it is and what level of risk is involved.
All medical device labels must include the name and address of the manufacturer, packer or distributor, and directions for use. Beyond these general requirements, medical device companies must determine the intended use and risk classification of their device to pinpoint their specific requirements. The FDA has published detailed regulations in 21 CFR Part 801. In addition, electromechanical medical devices have labeling requirements defined in IEC 60601-1 Clause 7. The usability of the labeling is addressed in IEC 62366. Canada’s regulations can be found in Canadian Medical Device Regulations clause 21. Refer to Medical Device Directive Annex 1, clause 13 for EU regulations.
The Importance of Proper Medical Device Labeling
A medical device label is intended to educate users, namely patients or healthcare providers, on how a device should be used, what risks are involved, and how to safely operate and maintain the device. Given that many medical devices are used by the patient at home, not under supervision by a medical expert, it’s critical that devices give accurate information about how to use and interpret the device to avoid injury, illness, or death.
For instance, a prefilled syringe allowing a patient to administer a measured dose of medication at home could help treat or prevent a specific disease when used correctly. If used incorrectly, a number of adverse effects could occur, such as injury from improper handling of the needle or an incomplete dose of medication. Because of these scenarios, a thorough analysis of human factors and usability testing is necessary to ensure device labeling covers all bases.
The Do’s and Don’ts of Medical Device Labeling
Given how lengthy and varied medical device labeling requirements can be, the process can be tricky for medical or pharmaceutical companies attempting to create this labeling. Tips for what to do and what to avoid are outlined below. Note that each jurisdiction has its own requirements, so be sure to check regulations for each country or region where you plan to commercialize the device.
What to Do
When labeling medical devices, do:
- Include labeling early on in your design planning
- Identify the device by make, model number, date of manufacture, and batch or serial number
- Provide contact information for the manufacturer and/or the local representative or distributor
- Include the intended use and indications for use and any contraindications or possible side effects
- List the box contents, meaning everything supplied with the device
- Include instructions for correct usage
- Include any warnings or cautions resulting from the risk management process
- Use appropriate symbols where possible in the labeling of the device
- Select label materials that will remain legible for the life of the device and are compatible with cleaning and sterilization methods defined in the instructions for use
- Have someone with regulatory knowledge review the labeling before submitting the device for clearance
What Not to Do
The following should be avoided on medical device labels:
- Including any text which contradicts the intended use or describes or refers to an off label use
- Including any marketing statements that are not supported by data
- Using the certifying body (e.g. CSA, ETL, UL) artwork on the product label without written permission
- Making any labeling changes after the device has gained regulatory clearance
Learn more from Gilero in these posts: FDA 510(k) Submission Process: An Introduction & How-To Guide and Combination Products: Definition & Overview.
Gilero is a trusted partner for medical and drug delivery devices from start to finish. For more information about our services and capabilities, contact Gilero today.