Human Factors for Medical Devices
As medical and drug delivery devices grow increasingly complex, regulatory agencies continue to place a greater emphasis on ensuring the safety and effectiveness of these devices through user research and human factors testing. Anyone using a medical device, whether clinician or patient, should be able to do so without making mistakes that could compromise the safety of the end user.
Human factors studies examine how a user interacts with a device in a simulated use environment. The goal of human factors and user testing in the medical device industry is to identify and mitigate any avoidable errors that could occur by considering the user, environment, and interface in relation to the product. These simulated scenarios could try to emulate a surgeon in an operating room, a nurse in a clinical setting, or a patient at home with a connected medical device. Human factors and usability testing for medical and drug delivery devices is crucial to ensure that the product design is readily embraced by users and can be operated safely in real-world conditions.
Requirements & Steps for Human Centered Design
An obvious requirement of designing a new medical device, or any type of device, is that it must function as intended. Human-centered design asks questions beyond ‘does this device work the way it’s supposed to?’ and considers whether users can successfully operate the device in the way it is intended to be used. Do the instructions for use that come with this medical device make sense to the clinician who will be operating it? Does the method for using this drug delivery device fit into a pharmacist’s current workflow? Can users properly manipulate the device or modify settings? Incorporating human factors engineering early within the medical device design process is essential for reducing use-related errors and risks.
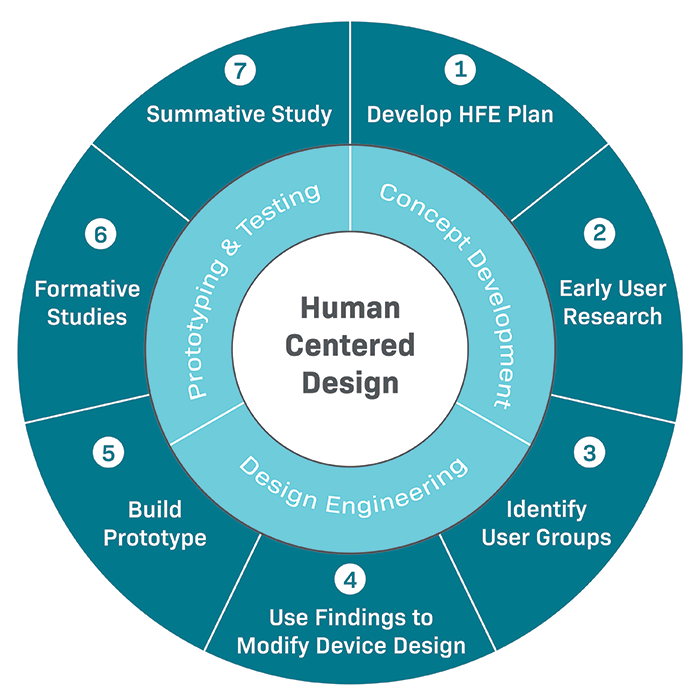
Steps for Applying Human Factors & Usability Testing to Your Medical Device:
Human factors and usability engineering can be executed differently depending on the device and its requirements. At a high level, the steps for human factors in the medical device industry are as follows:
- Develop a Human Factors Engineering (HFE) plan
- Gather early user research (this could include contextual inquiry, individual interviews, focus groups, surveys, etc.)
- Identify distinct user groups, understand their needs, and try to anticipate how they will interact with the device
- Apply findings from user research to modify device design and create a prototype
- Execute formative studies with several people from each user group (5-9 per distinct user group)
- Make necessary changes to the device based on results, then execute additional formative studies as needed
- When the medical device and labeling are close to finalized, complete a validation study with at least 15 individuals from each significant user group
For US markets, the FDA strongly suggests submitting a HFE/UE report summarizing human factors testing activities when a 510(k) is submitted for a medical device. It’s important to review the FDA guidance documents for applying human factors and usability engineering to medical devices and combination products to ensure the testing plan meets certain criteria.
Human Factors Engineering Outputs that the FDA Will Review
Regulatory bodies, such as the FDA, specify a series of activities that should be performed to ensure that your product is safe and effective for use once it hits the market:
- Research & document intended use, users, use environments, and use scenarios
- Document use-related risks, identify potential hazards, hazardous situations, and harms from foreseeable misuse, and specify design controls for anything that could lead to harm
- Develop user interface specifications (requirements)
- Conduct iterative evaluations of the device throughout development, to identify ways to improve the usability and safety of the device
- Execute a human factors validation (summative) study to demonstrate that the device is safe for its intended use, the intended users, and the intended use environments
- Generate an HFE/UE Summary Report to support the final submission
Each step in this process can use a variety of different research, analysis, and testing activities. Some activities, such as Task Analyses, are almost always performed. Others, such as HF Validation studies, are only needed when your device meets a certain safety threshold.
The Basics of Human Factors Research & Studies
When folks hear “human factors,” the first thing that usually comes to mind is usability testing. This is completely understandable, as usability testing is probably the most visible part of HFE/UE, not to mention the most entertaining!
At the highest level, studies fall into two categories:
- Formative Studies – Formatives are for learning what you don’t yet know. The purpose of a formative is to identify ways to improve your design – in particular, the design of your user interface, but also its instructions, packaging, and even training. Formatives are for finding out where your design is on the right track, and where it is off track. They give you a controlled, low-stakes way of evaluating the progress of your design.
- HF Validation (or Summative) Studies – HF Validation studies are for confirming what you already know. The purpose of an HF Validation study is to get data that proves that your device is safe and effective for use.
Other Types of Human Factors Studies:
While those two are the most familiar types of HF studies, there are many other kinds of studies as well. These include:
- Contextual Inquiry / Ethnographic Studies / Cognitive Walkthroughs – Generative studies that involve learning about users, use environments, and user workflows. These can be quite valuable as an input to early design efforts.
- Concept Evaluation Studies – Exploratory research that involves presenting intended users with one or more early concepts. This is a good way to get good qualitative data on what users think about your ideas, and how they might imagine it fitting into their workflows. With this information, you can justify choosing a leading concept, or which features of different concepts are ideal.
- Anthropometric studies – Research involving the physical attributes or limitations of your intended users. Literature does not always provide the specific data that you need to make design decisions, so occasionally you may need to generate your own. For instance, if you’re making an injector that’s designed to be used by individuals who have hand deformities, and you’re wondering what the ideal actuation force of the main button should be, you might need to get data on the actual hand dexterity and strength of these users.
- Label Comprehension studies – Studies that specifically evaluate whether end users can locate, interpret, comprehend, and apply the critical information on your labeling and instructions-for-use.
Risks Associated with Human Factors in Medical Device Design
Human Factors Engineering (HFE) is a critical aspect of medical device design, but it also poses certain risks. Some of the risks associated with HFE in medical device design include:
- Design-related errors: If the device is not designed with the user in mind, it can be prone to human misuse, which can lead to harm to the patient.
- Misinterpretation of information: If the information presented on the device or in the user manual is difficult to understand, users may make incorrect decisions that can lead to harm.
- Inadequate training: If users are not properly trained on how to use a device, they may not know how to use it safely and effectively.
- Inappropriate use: If a device is not designed with the user’s needs and limitations in mind, they may use it inappropriately and cause harm to themselves.
- Usability issues: If the device is difficult to use, it can cause frustration or error, resulting in the patient not getting the intended outcome of the device.
- Poor communication: If the device does not facilitate effective communication between healthcare providers, patients, and caregivers, it can lead to errors and misunderstandings.
- Failure to consider environmental factors: If the device is not designed to account for the environment in which it will be used, it can lead to harming the patient. For example, a device designed for a hospital setting may not work properly for at-home use.
It is important for medical device designers to identify and address these risks via human factors engineering throughout the design process. This includes conducting usability testing and incorporating user feedback to ensure the device is safe and effective for its intended use.
Choosing the Right Human Factors Partner
When human lives are at stake, human factors testing is an invaluable investment. If you ignore human factors, you put your users and patients at risk, and regulatory bodies such as the FDA will not approve your product for the market. If you perform human factors but do it poorly, you may end up on the market, but your development costs go up, while investments, sales, and adoption rates go down, putting your business objectives at risk.
At Gilero, human-centered design is a core value. Our in-house team of human factors experts are knowledgeable and experienced in all aspects of medical device usability engineering. Whether your device is simple or complex, or whether you need help with a simple formative study or help with an entire engineering effort, Gilero can help guide you through the design and development process.
For more information about incorporating human factors & usability engineering into your next project, contact us, today!
Ready to turn your idea for a medical or ocular drug delivery device into a reality?
Talk with an expert today.


