5 Ways to Ensure a Smooth Design Transfer Process
An effective design transfer is critical for medical device manufacturing. When done well, the process is faster, the cost is more economical, and the final product is more likely to meet all design specifications. Done poorly, production is likely to be slowed down, unexpected costs arise, there may be gaps in the supply chain, and the end result isn’t what was expected. But before we go into detail about what it takes to ensure a smooth design transfer process, let’s first understand what design transfer is.
What is Design Transfer for Medical Devices?
Design transfer is the process of providing guidelines to transition a medical device design from the design and development stage into manufacturing. This involves making sure the manufacturer will be able to realistically and successfully produce the medical device by establishing procedures that ensure the device complies with regulatory requirements and industry standards. Here are five practical tips you can utilize for a more successful transfer from design to manufacturing.
What are the Steps in the Design Transfer Checklist?
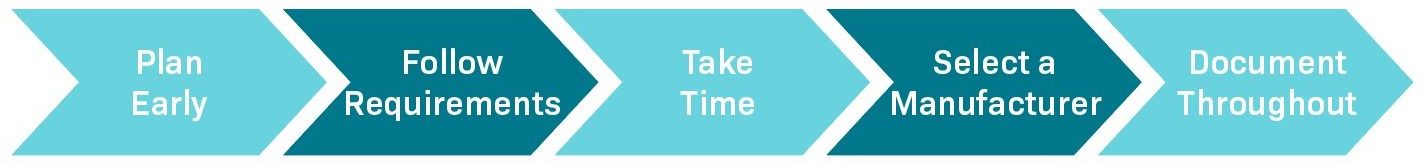
When it comes to a design transfer there are a few key things to keep in mind for implementation. The first is proactivity, as considering manufacturing from the start will save time in the long term. Secondly, the requirements for the transfer should be clearly laid out and strictly adhered to. This will make the project more efficient, however, it’s important not to rush. The third thing to consider is how long it will take to properly work through the process and to allow enough time for that. Next, it’s critical to partner with a trusted manufacturer who will get the job done correctly. Finally, make sure to document everything every step of the way so that the information included will be accurate and timely.
1. Think About Transfer to Manufacturing from the Beginning
If you don’t consider manufacturing until a product is nearing the end of development, you can be almost certain you’ll run into manufacturing design transfer issues. Medical device designs that only take into consideration product needs or user experience with no foresight as to how the design will be produced will inevitably lead to manufacturing delays or costly design changes. The items you design have to be manufactured again and again. This process must be efficient and cost-effective, especially in the medical device industry.
Don’t simply assume that the product development team will design for manufacturing. Involve manufacturing engineers or production team leaders in the design process to ensure the design can be reasonably manufactured at the necessary volumes. These experts can assist with design transfer by determining a timeline, sourcing components, explaining options for approaching production, and offering solutions for challenges that may be faced. Involving them early in the design phase can prevent wasted time and money during medical device manufacturing.
2. Clearly Designate and Follow Requirements for Design Transfer
The requirements set forth for medical device manufacturers in 21 CFR 820.30 state that translating the design of a device into product specifications is critical to device quality. The level of detail required in those specifications depends on the device, the relationship with the manufacturer, and the skill of production workers. This information should be captured in a product development plan that contains all necessary documentation and is supported by verification and validation records. Having a detailed product development plan will lead to a smoother design transfer of the medical device. Have these specifications properly reviewed and approved to ensure they are precisely what the manufacturing team should follow, and ensure that all requirements are being met on time.
3. Don’t Rush the Process
The production process can be lengthy, particularly when a device design is complex or utilizes new manufacturing technology. After an extensive design and development process, you likely want to get your device through production and into the hands of consumers as quickly as possible. However, taking the time to follow established, well-executed procedures is a key component of successful medical device manufacturing. Avoid putting any aspect of the process in jeopardy by giving everyone involved plenty of time to understand all requirements and do their part properly. Try to account for potential unforeseen delays, such as supply chain inefficiencies, and eliminate common causes of them. Getting things right in the design transfer stage can help set you up for long-term manufacturing success.
4. Partner With the Right Manufacturer
There are several factors to consider to find the right manufacturer for your needs. When transferring a medical device from design to manufacturing, you need a manufacturer that knows and is prepared to follow FDA guidelines and Good Manufacturing Practices (cGMPs). Also, consider industry experience. Selecting a manufacturer with a proven track record of success completing medical device projects will help eliminate design transfer issues down the line. The manufacturer you work with should also have a quality management system in place and be able to fulfill the volume of production needs now and as you scale up.
5. Document as You Go
Don’t wait until you’re far into the process to start creating design transfer documentation. You’ll be able to create much more thorough and useful reports if you document at every stage. A good rule of thumb when working with medical devices is to properly document everything, in real-time, and use a trusted Quality Management System (QMS) to keep track of this documentation. If additional elements are developed or evolve over time, you’ll avoid confusion and frustration if you document as you develop. Having a full, documented process for transferring a design to manufacturing makes it less likely that mistakes will happen.
Medical Device Design Transfer at Gilero
Gilero provides design, development, and contract manufacturing services for the medical device and pharmaceutical industries. Our manufacturing expertise ranges from single-use medical devices to complex electromechanical drug delivery systems. Manufacturing service offerings include cleanroom manufacturing, plastic injection molding, injection mold tool building, complex assembly, packaging and kitting. With a proven capacity to innovate, scale, and commercialize, our domestic and international facilities are equipped to develop unique manufacturing solutions and accelerate device commercialization timelines. Contact Gilero today to learn more about our contract manufacturing services, including design transfer.
Ready to turn your idea for a medical or ocular drug delivery device into a reality?
Talk with an expert today.


