Medical Device Design & Development: An Informative Guide

Medical devices make medical intervention easier and more successful. They range from tongue depressors and catheters to pacemakers and medical lasers. Medical devices have been used by clinicians for as long as medical intervention has existed, but within the last several decades, advances in medical device design & development have significantly pushed the industry forward, as well as improved the health and well-being of the people who use these devices. As the industry has progressed, connected medical devices are becoming more common with the rise of smart health solutions.
What is Medical Device Design?
Medical device design is the process of designing a device intended to be used for medical purposes. As medical device designers, our team of experts ensures that we follow the latest regulations and follow best practices. In general, there are a few key steps that take place during the medical device design process.
Defining Device Requirements
First, requirements for the device must be gathered and defined. In design & development projects, this step includes understanding each of the design constraints that define the design space. We often use these categories to characterize the design space: performance, manufacturability, Intellectual Property constraints, regulatory constraints, material requirements, cost of goods, aesthetics and usability. At Gilero, our design & development teams are generally led by a Project Manager and a Technical Lead. While the Project Manager is tasked with keeping the project on time and on budget, the Technical Lead must help define and subsequently operate within the boundaries of these requirements to design a successful product, not just a good-looking device.
Next comes medical device concept development. The technical, regulatory, business and other requirements that have been defined are taken into consideration during this early definition of white space. Product design engineers, working with cross-functional teams, brainstorm ideas and solutions to develop a design concept that will fulfill customer needs and meet the necessary product requirements.
Proof of Concept Phase
The next step in the medical device design process involves putting the design through a proof of concept phase to demonstrate that all critical features of the design are feasible and will function as intended. Prototypes will be developed to assess performance and address design challenges while evaluating the form, fit and function of the device. A proof of concept prototype intends to prove through testing that the design is feasible from both a technical and business standpoint.
Based on the outcome of early medical device prototyping and proof of concept testing, design revisions may be required. Once it has been determined the design concept is viable, the design is put under what we refer to as a concept freeze. A concept freeze means that no more significant changes will be made to the design.
This is where medical device product development truly begins. Some design work, such as Design for Manufacturability (DFM), or to prepare items such as test fixtures and prototype tooling, may continue throughout the development process, but the concept freeze signals that the product design is ready to transition from design to development.
What is Medical Device Development?
Medical device product development is the process of turning a medical device design into a commercially viable product. In the medical device industry, development engineers must follow the phases of medical device development, adhere to strict regulatory requirements and thoroughly document their work.
Design Planning – Design Inputs and Outputs
It starts with a planning phase. A design plan will be formally documented per regulatory requirements to outline the medical device product development process. The design inputs phase follows quickly thereafter. Requirements and specifications that were laid out during the design process must now be formally documented as user needs and product requirements in the design inputs phase. These first two phases define what mode(s) of testing will need to take place, what test equipment is needed, what processes must be followed, what documents need to be prepared and how the medical device will be manufactured.
In the Design Outputs phase, the designs that have subsequently reached concept freeze are then translated into engineering design specifications under Design Control procedures. Using CAD and 2D drawings, a detailed product specification will be created. At this point the device will be transferred in to manufacturing on a small scale so that devices for testing can be produced using commercially representative materials and methods.
Testing and Medical Device Design Verification & Validation
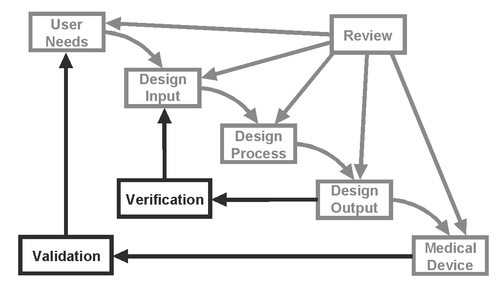
Testing will occur throughout the medical device product development process. Building upon early prototype iterations and engineering evaluations, formal test methods and protocols specific to the device will be generated, including a Design Verification and Validation (DV&V) plan. Design Verification and Validation is one of the most important parts of medical device development and happens late in the medical product development process. Design Verification will determine if the device was designed correctly and meets requirements, while Design Validation will demonstrate whether the right device was designed and if it meets user needs. Completed reports of testing activities will be used in regulatory submissions.
Although achieving regulatory approval is often seen as the last step in medical device development, regulatory planning should be considered early in the process. Different types of devices have different regulatory requirements, and regulatory rules can vary by country or region. Data and documentation surrounding development will need to be prepared, maintained, and submitted to the appropriate authorities for approval.
The Importance of the Medical Device Design and Development Process
Because medical devices directly affect the health and well-being of patients, designing and developing them will always carry some level of risk. There’s much more that goes into medical device design and development than simply coming up with an idea, building it, and offering it up to the masses; it’s a complex process that is filled with considerations regarding regulations, specifications, application requirements and user needs that must be carefully followed in order to produce a safe and effective product that will succeed in the market.
Design & Development Regulation and Risk Management
When designing medical devices that are intended to improve lives, it is important to make sure that risk to the end user is as minimal as possible. Regulatory authorities such as the Food and Drug Administration (FDA), European Medicines Agency (EMA), and other international counterparts have created a broad range of regulatory documents and risk management procedures to ensure that safety measures are taken at all steps of medical device development and production. The International Organization for Standardization (ISO) is a non-governmental, global organization that develops standards ensuring the quality, safety, and efficiency of products, services, and systems. A few of the most common ISO standards for medical device risk management include:
- ISO 13485 specifies requirements for the quality management system (QMS) that can be used by medical device developers and manufacturers. This standard offers guidance for medical device companies to help ensure the highest level of safety and quality for these products.
- ISO 14971:2007 is specifically used to identify the risks associated with medical devices and how to estimate, evaluate and control them.
- IEC 60601-1-2 relates to ensuring the basic safety and essential performance for electromechanical medical equipment is maintained in the presence of electromagnetic energy.
These are the primary standards, but there are a wide variety of others that must be considered when designing a medical device. In terms of managing risk, there are a couple different techniques employed including Hazard and Operability Study (HAZOP) and Failure Mode and Effects Analysis (FMEA).
- HAZOP is a study done to examine and analyze existing processes or operations in order to identify any potential problems or risks associated with the medical equipment design.
- FMEA is a structured approach to review all components of a device, searching for any part of the system which could potentially fail, and analyze the risk associated with these failures.
If you need help with medical device design and development, contact Gilero today!
Ready to turn your idea for a medical or ocular drug delivery device into a reality?
Talk with an expert today.


