Tony Stark, Ironman & Medical Device Design
I always thought that everything I needed to learn I could learn from The Simpson’s but recently I have had to turn to comics and superheroes to learn about designing a battery powered medical device. In this case I’m referring to Ironman. Tony Stark had an electromagnet implanted in his chest to keep pieces of metal shrapnel that were lodged in his chest from entering his heart and killing him. This was great except the electromagnet was powered by a large car battery. It was impractical, difficult to carry around and the limited capacity of the battery almost killed him and he had to come up with a new design to power the device. This led him to implanting an Arc reactor in his chest and later becoming Ironman. Not a bad result in the end.
In all seriousness, battery-powered and smart medical devices are becoming more and more abundant as they provide improved accessibility, mobility, point of care use, and home health care opportunities. With the advancements in battery technologies allowing for greater charge capacities and longer runtimes the decision to go wireless seems like a no brainer. However, battery powered medical devices can have serious consequences if not designed correctly. They could potentially cause severe harm to a patient or even death. There are many safety risks associated with battery powered medical devices but probably the biggest risk is a failure of the device to provide proper therapy to a patient. This can happen for numerous reasons like bad voltage monitoring, improper charge or discharge, loss of data or data retention, shortened lifespan, environmental factors like temperature, fluid ingress, shock and vibration. In addition, the battery themselves pose potential risk from electric shock, overheating, swell, or even ignition and fire.
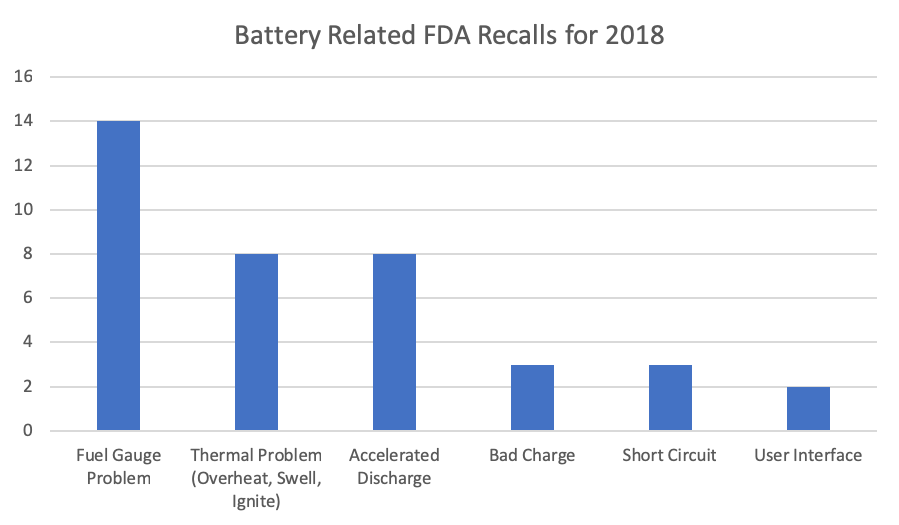
Searching through the FDA database for Medical Device Recalls (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm#) shows there were 38 recalls in 2018 for battery related issues, 28 in 2017, 25 in 2016, and 53 in 2015. This may not seem like a lot of recalls but it does show that there is a problem. This number is surely going to keep rising as the number of battery powered devices on the market increases. All of the recalls from 2018 were Class II recalls except for one Class I recall. Class I recalls are the most severe in which a product could cause serious adverse health consequences or death. Class II recalls may cause temporary or medically reversible adverse health consequences. Even though the majority of these recalls are Class II and pose less risk to the user they should not be taken lightly. The following chart shows the categories of the battery related recalls from 2018.
There are many steps and challenges in designing a battery powered medical device. The battery cell chemistry, type, voltage, capacity, and shelf life need to be specified as well as the operating environment. If using a rechargeable battery, the charge and discharge characteristics are crucial to the design. There are also mechanical design considerations, service life, safety features, user interface, transportation, regulatory compliance, and manufacturing requirements as well.
The charge/discharge characteristics and the fuel gauge of the system provide the biggest design challenge especially in multi-cell battery designs. Rechargeable Lithium Ion batteries are more sensitive to overcharging, overheating, over-discharge and improper charge levels than other battery cell chemistries. In multi-cell batteries, the individual cells behave slightly differently, and this needs be taken into account when designing a medical device. During use the battery discharge should stop when the cell with the lowest capacity reaches its safe lower voltage limit to prevent over-discharge. In addition, the system should stop charging when the cell with the highest capacity reaches its high limit to prevent overcharging of the cells. Consequently, this will limit the overall capacity of the battery. A balancing circuit can help overcome some of these short falls by charging or discharging the cells individually. Active or passive battery balancing circuits help to safely utilize the full battery capacity. In addition to a balanced charger a proper fuel gauge can help to accurately measure the state of charge of a battery. In addition to improving the overall lifespan, reliability, and runtime, knowing the state of charge is paramount for the proper operation of a medical device.
We’ve all experienced it with our cell phones. You check the battery and it shows 75% battery left so you don’t worry about charging it and go on with your day. Next thing you know your phone is showing 5% left and start scrambling to find a charger. Luckily for us though, nothing tragic will happen if our phone battery dies. We might just miss out on a few emails or a few updates on social media. Now imagine if that happened with your insulin pump or pacemaker. The outcome would be less than desirable. However, adding a balanced battery charger and accurate fuel gauge can overcome these pitfalls but come with the burden of added complexity and cost to the overall design.
Another big factor that affects the operation of batteries is the operating environment. Normally most medical devices are designed to be used in nice climate-controlled environment like a hospital or healthcare facility. Now that the devices are becoming battery powered they are being exposed to extreme temperatures. Temperature has a huge impact on the operation and life of a battery. Therefore, defining and testing the true operating environment of the device up front will help prevent early battery replacement or worst case, explant of an implanted medical device which can lead to further injury or health complications to the patient.
There is a large discrepancy in life expectancy between battery, 3-5 years at best and medical device 7-15 years. This means that the battery will have to be replaced at some point. This can be done by a user or trained service person but either way more effort needs to go into the design to ensure safe replacement and proper disposal of the battery. It may seem trivial but two of the FDA recalls in 2018 were related to the User Interface including improper instructions for replacing the battery.
Batteries should not be seen as a commodity part of a medical device nor should the design effort be underestimated. Proper design will lead to safe and effective battery powered medical devices that will help expand our knowledge and improve our health as it will open up doors to new research and care that were once limited by the need to be connected by a cord to a power source.
The author of this article, Jeff Carroll, is a Senior Electrical Design Engineer at Gilero.
Ready to turn your idea for a medical or drug delivery device into a reality?
Talk with an expert today.


